ABSTRACT
The effect of immersion plating deposition of Cu and Ag metals onto a thin layer of stain etched porous silicon on its photoluminescent properties has been investigated.
FTIR and photoluminescence spectra of samples before and after deposition were analyzed. It was found that the deposition of these metals leads to quenching of the
photoluminescence, which is not restored even after removal of these metals by acid etching. FTIR analysis shows that the quenching of the photoluminescence correlates with
the disappearance of the absorption band in the 2000-2300 cm-1 range corresponding to hydrogen vibrations, and the appearance of a new absorption peak at 1221 cm-1, that is
not typical for porous silicon. It was found that this absorption peak appears under the influence of the deposited metal, although the metal itself does not participate in
the corresponding bonds, since this peak remains even after the removal of the deposited metal by acid etching. The ability of porous silicon to instantly dissolve in
dilute HF after metal deposition, as well as the proximity of the peak at 1221 cm-1 to the stretching vibrations Si-O-Si at 1172 cm-1, suggests a relationship between this
peak and oxygen vibrations. The correlation of photoluminescence quenching with the occurrence of an absorption peak at 1221cm-1 indicates the deformed nature of these
oxygen bonds. The deformed bonds lead to the formation of nonradiative recombination centers, which cause the quenching of photoluminescence.
Keywords: porous silicon; metal deposition; photoluminescence; FTIR, hydrogen bonds; oxygen bonds
DOI:10.70784/azip.1.2025226
Received: 09.04.2025
Internet publishing: 29.04.2025 AJP Fizika E 2025 02 en p.26-31
AUTHORS & AFFILIATIONS
Condensed Matter Physics Division, Institute for Physical Problems, Baku State University, Baku AZ 1148, Azerbaijan
E-mail: farhad.rustamov@bsu.edu.az
Graphics and Images
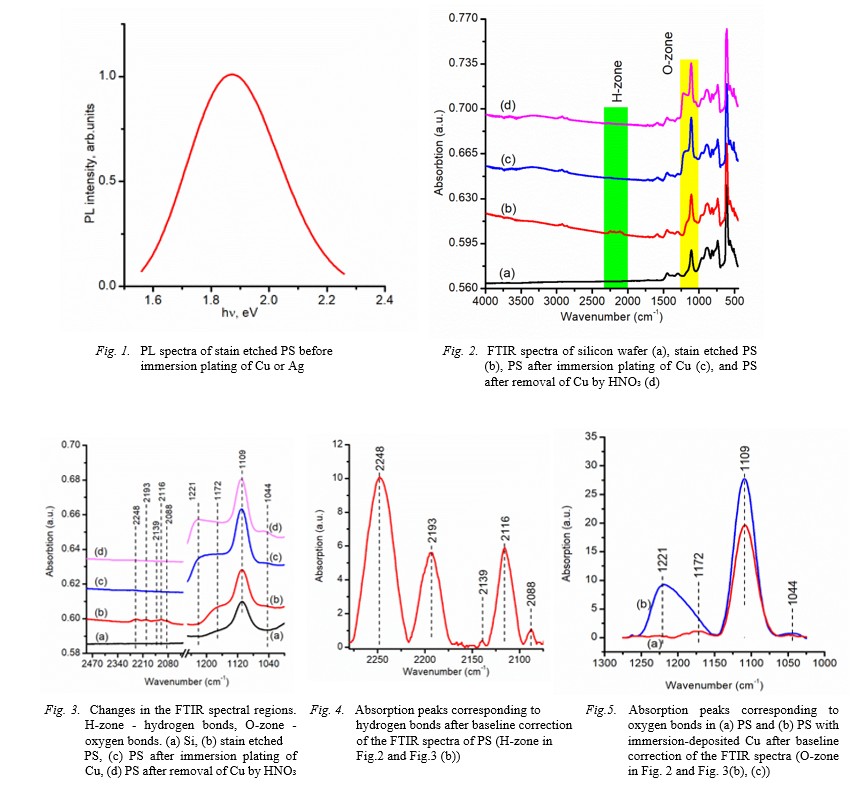
Fig.1-2-3-4-5
|
[1] L.T. Chanham. Properties of Porous Silicon. 1997, EMIS Data Review Series № 18, London
[2] V. Lehmann. Electrochemistry of Silicon. 2002, Willey VCH, New York
[3] M.J. Sailor. Porous Silicon in Practice. Preparation, Characterization and Applications. 2011, Wiley-VCH, Weinheim, Germany
[4] D. Andsager, J.M. Hetrick, J. Hilliard, M.H. Nayfeh. Diffusion of copper in porous silicon. J. Appl. Phys. 1995, 77(9), 4399-4402. https://doi.org/10.1063/1.359606
[5] A. Cetinel, N. Artunc, E. Tarhan. The growth of silver nanostructures on porous silicon for enhanced photoluminescence: The role of AgNO3 concentration and deposition time. Eur. Phys. J. Appl. Phys. 2019, 86(1), 11301p1-11301p9. https://doi.org/10.1051/epjap/2019190013
[6] D. Andsager, J. Hilliard, J.M. Hetrick, L.H. AbuHassan, M. Plisch, M.H. Nayfeh. Quenching of porous silicon photoluminescence by deposition of metal adsorbates. J. Appl. Phys. 1993, 74(7), 4783-4785. https://doi.org/10.1063/1.354350
[7] F.A. Harraz, T. Tsuboi, J. Sasano, T. Sakka, Y.H. Ogata. Metal deposition onto a porous silicon layer by immersion plating from aqueous and nonaqueous solutions. J. Electrochem. Soc. 2002, 149, C456-C463. https://doi.org/10.1149/1.1498841
[8] J.E. Hillard, H.M. Nayfeh, M.H. Nayfeh. Re-establishment of photoluminescence in Cu quenched porous silicon by acid treatment. J. Appl. Phys. 1995, 77(8), 4130-1995. https://doi.org/10.1063/1.359559
[9] K.Y. Suh, Y.S. Kim, H.H. Lee. Blue photoluminescence from in situ Cu-doped porous silicon. J. Appl. Phys. 2002, 91(12) 10206-10208. https://doi.org/10.1063/1.1476961
[10] Y.M. Huang. Photoluminescence of copper-doped porous silicon. Appl.Phys. Lett. 1996, 69(19), 2855-2857. https://doi.org/10.1063/1.117341
[11] Y.W. Lu, X.W. Du, J. Sun, X. Han. Influence of surface Si-Ag bonds on photoluminescence of porous silicon. J. Appl. Phys. 2006, 100, 063512-1 - 063512-4. https://doi.org/10.1063/1.2353397
[12] X.I. Song, D.Y. Xu, H.P. Yang, Z.X. Yu, G.Z. Qiu. Ag deposition forms and uniformity on porous silicon by electrochemical method. Chin. J. Chem. Phys. 2010, 23(2), 211-216. https://doi.org/10.1088/1674-0068/23/02/211-216
[13] T.D. Dzhafarov, A.H. Bayramov, S.X. Ragimov, A.R. Imanaliyev, E.A. Bagiyev, A.M. Karimova, S.G. Asadullayeva, J.N. Jalilli. SnO/porous silicon heterostructure based humidity sensor. AJP FIZIKA. 2022, pp 41-45
[14] D. Gräf, M. Grundner, L. Mühlhoff, M. Dellith. Influence of Cu on the native oxide growth of Si. J. Appl. Phys. 1991, 69(11), 7620-7626 https://doi.org/10.1063/1.347531
[15] É. Vázsonyi, E. Szilágyi, P. Petrik, Z.E. Horváth, T. Lohner, M. Fried, G. Jalsovszky. Porous silicon formation by stain etching. Thin Solid Films. 2001, 388(1–2), 295-302. https://doi.org/10.1016/S0040-6090(00)01816-2
[16] F.A. Rustamov, N.H. Darvishov, M.Z. Mamedov, E.Y. Bobrova, H.O. Qafarova. Formation of lateral homogeneous stain etched porous silicon with acetic acid at oxidant insufficiency. AJP Fizika. 2012, 18(3), 44–49
[17] F.A. Rustamov, N.H. Darvishov, V.E, Bagiev, M.Z. Mamedov, E.Y. Bobrova, H.O. Qafarova. Influence of final treatment on the incubation period and antireflection properties of stain-etched porous silicon. Phys. Status Solidi A. 2013, 210 (10), 2174–2177. https://doi.org/10.1002/pssa.201329298
[18] F.A. Rustamov, N.H. Darvishov, V.E. Bagiev, M.Z. Mamedov, G.M. Eyvazova, E.Y. Bobrova, H.O. Qafarova. Reversible quenching of photoluminescence in stain etched porous silicon at HNO3 posttreatment and role of oxygen bonds. J. Lumin. 2018, 195, 49-53. https://doi.org/10.1016/j.jlumin.2017.11.011
[19] M.J. Winton, S.D. Russell, J.A. Wolk, R. Gronsky. Processing independent photoluminescence response of chemically etched porous silicon. Appl. Phys. Lett. 1996, 69, 4026–4028. https://doi.org/10.1063/1.117859
[20] E. Kayahan. The role of surface oxidation on luminescence degradation of porous silicon. Appl. Surf. Sci. 2011, 257(9), 4311–4316. https://doi.org/10.1016/j.apsusc.2010.12.045
[21] B. González-Díaz, R. Guerrero-Lemus, J. Méndez-Ramos, B. Díaz-Herreraa, V.D. Rodríguez. Gradual oxidation of stain etched porous silicon nanostructures applied to silicon-based solar cells. Sens. Actuators A. 2009, 150, 97–101. https://doi.org/10.1016/j.sna.2008.12.006
[22] Y.Q. Jia, L.Z. Zhang, J.S. Fu, B.R. Zhang, J.C. Mao. Characterization of stain etched porous Si with photoluminescence, electron paramagnetic resonance, and infrared absorption spectroscopy. J. Appl. Phys. 1993, 74(12), 7615–7617. https://doi.org/10.1063/1.354940
[23] A.E. Surkhayli, H.A. Shirinova, B.G. Pashayev, A.H. Karimova, S.G. Nuriyeva, L.R. Gahramanli. Investigation of infrared and Raman spectra of polymer nanocomposites based on polycrystalline silicon nanoparticles. AJP FIZIKA E. 2024, XXX, № 4, pp. 17-22. DOI:10.70784/azip.1.2024417
[24] V.P. Tolstoy, I.V. Chernyshova, V.A. Skryshevsky. Handbook of Infrared Spectroscopy of Ultrathin Films,.John Wiley & Sons, Inc, Hoboken, New Jersey, 2003.
[25] J.H. Song, M.J. Sailor. Dimethyl Sulfoxide as a Mild Oxidizing Agent for Porous Silicon and Its Effect on Photoluminescence. Inorg. Chem. 1998, 37(13), 3355-3360. https://doi.org/10.1021/ic971587u
|
